(How) should we pursue human longevity?
Unlike human beings, the debate over the question “should people live forever, if they want?” never seems to die.
This is surprising given that the answer is obviously “yes.” In fact the arguments for “no” are so unimpressive I’m relegating them to an Appendix.
A better question is “should I work on human longevity, and if so, how?"1
This is a personal question, but answering it well involves a shedload of strategic and economic considerations. I couldn’t find a comprehensive guide to them, so here’s my attempt at one.
Contents
Tractability and Strategy
Y Combinator sums up the opinion of the tech world today:
“We now have the understanding of biology to work on therapies that directly address the root causes of aging.”
Is this true? And if so what’s the best way to go about it? There are (at least) three big considerations.
Do we know the causes of aging, and do we even need to?
I’d say “not yet” and “maybe not.”
Here’s a summary of what experts think causes aging in mammals and how they figured them out.
| Proposed cause for aging | Brief timeline of discovery | Refs |
|---|---|---|
| Nuclear DNA damage accumulation | Free radical theory of aging proposed in 1956. Strong evidence in 1990s that DNA damage leads to aging. Still not confirmed that DNA repair leads to longevity. | 1 |
| Stem cell exhaustion/cell loss | Stem cells discovered 1963. Causal connection between stem cell exhaustion and aging first demonstrated in 2012. | 1, 2 |
| Senescent cell accumulation | Replicative senescence described by Hayflick in 1965. Oncogene-induced senescence described starting in 1980s. Stress-induced senescence described starting in 1990s. Tumor suppressor loss-induced senescence described starting in 2000s. Strong evidence of therapeutic potential in 2011. | 1, 2 |
| Mitochondrial DNA damage accumulation | Mitochondrial free radical theory proposed in 1972, now much less clear that it's correct. Non-ROS damage hypothesis made in 2005, evidence still accumulating. | 1, 2, 3 |
| Inflammation | Systemic inflammation/interleukins discovered in 1977. "Inflammaging" coined 2000. Causality still being worked out. |
1, 2 |
| Telomere attrition | Discovery of telomere structure and speculation about connections to replicative senescence in 1980s. Telomere loss hypothesis for aging proposed in 1991. Experimental evidence confirming connection in 1998. | 1, 2, 3 |
| Loss of intracellular proteostasis (chaperone and autophagy system decline) | Evidence starting to accumulate by 1983. Demonstration of causal role of chaperone system in mice in 2008. Demonstration of causal role for autophagy in mice in 2013. | 1, 2 |
| Extracellular aggregates | Amyloid known since 1800s. Connection with Alzheimer's proposed in 1991. | 1, 2 |
| Reduced IIS | daf-2 discovered in 1992. IIS pathway implicated in 1997. Causality still being worked out. | 1 |
| Epigenetic alterations | Heterochromatin loss model of aging proposed in 1997. Evidence accumulates for chromatin structure disruption at all levels in 2010s. | 1, 2 |
Nintil’s Longevity FAQ is a great starting point to understand the biology behind most of these proposed causes if you’re unfamiliar.
Let’s first admit that it’s dubious to break down aging into discrete causes like in the table above. Here’s a diagram giving a much more detailed view of our current understanding of aging-related processes. (In fact that diagram isn’t even complete since aging varies between different parts of the body at different times.) Based that diagram (seriously, click the link, it’s worth it), understanding the causality of aging may be less about discovering new causes and more about disentangling what we already semi-understand, if that’s even possible. Though it might still be true that there really are only a few root causes of aging behind all these.
Nevertheless, from the outside view the above table doesn’t really suggest that we’ve finished discovering fundamental causes of aging. The pattern — if small-N history can have patterns — seems to be that every decade a couple biological processes are associated with aging and then 1+ decades later, if ever, a causal connection is established. The table only includes causes of aging that have made their way into big general reviews, so we wouldn’t expect to, and don’t, see any with initial discovery dates past 2000. Biological processes whose heydays came after 2000 that might someday make it into this table include microbiome changes and RNAi dysregulation.
You might argue that since investment into aging research and biotech in general has increased over the years, but the rate of discoveries hasn’t changed, that means we’re nearly done discovering things. But, while we clearly know more about aging than we ever have, we still don’t have much of a causal understanding of aging.
But how much do we need to understand?
The standard playbook for making a new therapeutic (the catch-all name for a drug or medical treatment) is called a target-based approach, and goes like this:
- Identify a specific biological process that’s causing the disease you want to treat (e.g. plaque buildup, the expression of a single gene, a protein key to a virus’s function)
- Develop a way to interfere with it (e.g. a synthetic molecule, an engineered antibody, a surgical intervention) in vitro or in an animal model
- Adapt that treatment so it’s safe and effective in humans
It’s not clear this standard approach is well-suited to longevity therapeutic development.
Target-based approaches work best when we thoroughly understand the cause(s) of a disease. Bacterial infections and single-gene disorders are good examples: we really know what biological process we’re trying to interfere with to get the outcome we want. But as discussed above, we don’t understand the causes of aging, and our current understanding it that it’s massively multi-causal.
An alternative to the target-based approach for therapeutic development is the phenotypic approach, which goes like this:
- Set up an experiment where you can cheaply try a gajillion treatments and find one that affects the phenotype you care about (e.g. the phenotype of living longer)
- Adapt that treatment so it’s safe and effective in humans
In reality all therapeutic development takes place on a spectrum between target-based and phenotypic. Some understanding of causality is necessary for restricting the space of treatments screened in phenotypic approaches. And many causes of phenotypes are discovered via screening approaches.
Therapeutic development today generally leans toward target-based. This is despite the fact that more first-in-class small-molecule drugs are developed with phenotypic approaches. And despite the fact that many effective longevity therapies (in mice) had no mechanistic hypothesis when they were discovered, including parabiosis (and potentially neutral blood exchange) and caloric restriction.
Phenotypic approaches also seem better suited to finding effective combinations of therapies, for the inelegant reason that biological systems are so interconnected that to find an effective combination therapy we just have to try a lot of combinations. This is important for longevity, since it’s not clear how much partial credit we get for solving any single cause of aging. It may be that each one cured buys us more lifespan and eventually we reach longevity escape velocity. But since dying only requires one cause of death, we may need therapies that simultaneously address all or most causes of aging — even ones we don’t know of yet — before seeing benefits. The idea that phenotypic approaches might be better for targeting multi-causal biological phenomena like aging is not new. Companies working on them (not specifically on longevity) include Recursion, Janssen, and Insitro.
Phenotypic screening has plenty of challenges, of course. One is the gajillion factor: you need a way to try lots of different treatments, and the space of possible treatments is astronomical. Another is that you can’t do a phenotypic screen without a phenotype, and waiting for animals to die is pretty inefficient. Developing biomarkers (a.k.a. quantitative phenotypic measurements) for aging, like epigenetic clocks, is critical to enable better phenotypic screens. Gordian, Nemalife, Magnitude, InVivo, Vium, and Spring are among the companies working on these problems.
Do we have the technology?
It seems inarguable that humans can live forever in the limit of arbitrarily fancy technology, though some disagree.2 But whether death is conquerable not just eventually, but with near-term tools and understanding, is not clear. To paraphrase Lincoln: we don’t know whether we’ve sharpened the biotechnological axe enough to cut down the death tree.
This is a key question for longevity strategy. The more confident we are that existing therapeutic technologies can make a dent in aging, the more we should invest in using them. The less confident we are, the more we need to make new tools.
One relevant datapoint is what modalities (a.k.a. types of therapeutics) are being used by companies currently working on longevity. As far as we know, it’s about 51% small-molecule drugs, 16% biologics, 18% cell therapies, and 15% gene therapies. This skews more toward non-small-molecule therapeutics than the industry average, which is what we might expect given that most of the causes of aging in the table above don’t have obvious targets for small-molecule inhibition. It also suggests that new tools are needed, since non-small-molecule therapeutics are newer and less straightforward to develop than small-molecule drugs (as if those were straightforward to develop in the first place).
Another datapoint is what we’ve managed to accomplish so far in mice. Here’s a table mostly derived from a table of Laura Deming’s showing the greatest median lifespan increase that’s been achieved in mice by different therapeutic modalities.
| Modality | Greatest increase in median mouse lifespan achieved using this modality | Specific intervention that achieved it | Realistic modality for humans? |
|---|---|---|---|
| Spontaneous mutation | 68% | Ames Dwarf Mice | N |
| Germline gene therapy | 46% | GHRH knockout | N |
| Diet | 32% | Calorie restriction + lard | N |
| Small-molecule drug | 27% | Rapamycin | Y |
| Somatic gene therapy | 24% | Telomerase | Y |
| Biologic drug | 16% | Intranasal Hsp70 | Y |
| Cell therapy | 12% | HSC transplant | Y |
Note that median lifespan is not maximum lifespan, and lifespan often increased for only one sex of mouse.
The top two modalities in mice, spontaneous mutation and GGT, can’t be used (legally) in humans. And I’d argue diet is off the table for practical reasons; we’re talking extreme diets. The remaining modalities are used in some form in humans. I left surgeries off the table since it’s too broad a category, but the ones relevant to humans give <= 10% median lifespan increase. Also note that just because a lab chose to treat a mouse with one modality doesn’t mean another modality can’t accomplish the same effect. Developing small-molecule drugs to target proteins encoded by genes discovered by GGT in mice is standard practice.
You can read this table as showing that human-amenable modalities are less potent than they could be, or you can read it as a selection effect of what academics find easiest to do in the lab. The former is a big claim, and I’d need more evidence to believe it. The latter isn’t news to anyone. Synthesizing candidate drug molecules is slow. Producing immunotherapeutics is slow and hard. Gene therapy has a ways to go.
Altogether it seems clear that our therapeutic armamentarium is lacking, whether in potency or ease of use.
What are the biggest leverage points?
Bringing a medical treatment to market is extremely slow and expensive. It takes around a decade and $1 billion. A big reason for this is the 20% success rate for non-cancer drugs once they enter clinical trials, not to mention the definitely-less-than-100% success rate for making it to clinical trials in the first place.
Here’s a figure from this paper breaking down the costs, timelines, and success rates in a traditional target-based drug discovery campaign.
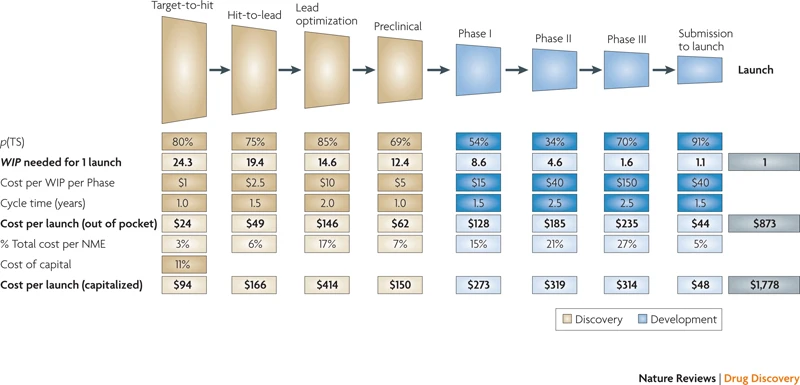
Paraphrased caption from the paper: Numbers are in millions of USD and based on data from Eli Lilly and Company. p(TS) is the probability of successful transition from one stage to the next. WIP is work in process, the number of candidate drugs being worked on. Lighter-shaded boxes show calculated values based on assumed inputs. Costs do not include investments for exploratory discovery research, post-launch expenses like marketing, or non-R&D overheads like non-scientist salaries.
Worth noting in the figure is that most of the cost is in the clinical trials (the blue parts), and that the step with the lowest success rate is Phase 2 clinical trials (the first time the therapy’s efficacy is tested in humans, usually).
What does this mean for longevity? Barring an adventure in regulatory arbitrage or medical tourism, any longevity treatments will have to get through this process before they can get to you or me.3 So it may well be that the best way to pursue longevity is to improve the drug development process, depending on how tractable and neglected various improvements are.
Diagnosing the problems in the drug development process is a lot like studying aging: everything you look at is broken, but we have no idea about the root causes or where best to intervene.
At the lab level
Animal models for disease are fraught with difficulty. Those for aging are particularly challenging. How challenging? So challenging that the longest-lived strain of mouse known to biology used to be used as a model for rapid aging, because the mice were being handled wrong and dying young.
Of course we could dispense with animals models and focus on human trials, but a trial that waits around for people to die will take a long time. See for example the TAME Trial, which plans to run for six years. As mentioned above, better biomarkers could help with this. Or one can follow George Church’s advice and ignore therapies for slowing down aging in lieu of those with undeniable, immediately noticable effects.
A big change in lab operations over the last two decades has been the “virtualization” of biotech, which is a fancy word for outsourcing. Work once done in-house, like synthesizing molecules or running toxicity assays, is now usually done by Contract Research Organizations (CROs). Outsourcing has benefits: increased specialization, lower prices, etc.
But outsourcing is also inefficient: CRO management is the biggest headache for many biotech companies. It’s still a lot easier to do drug development when everyone’s in the same building, or so says every scientist I’ve asked about it. This is not the case in all industries. Solutions could come in the form of automation — Emerald Cloud Lab and Transcriptic are two companies working on that — or better outsourcing offerings. One thing the field probably is good on is electronic lab notebooks. Thanks though.
At the community level
Longevity research, and medicine in general, is bad at integrating its knowledge and learning from failures. Between 25% and 50% of clinical trial results remain unpublished even several years after completion. This isn’t surprising: why publish your clinical data when there’s zero benefit to you for doing so, and when there’s always some chance you might want to repurpose the therapy in the future? There’s a similar market failure around improving animal models: animal models aren’t easy to defend with patents and are hard to keep secret, which means they get relatively little private R&D investment. All this means a potential downside of moving longevity efforts from academia to commercial therapeutic development is that the field may move more slowly.
A related problem is that because liquidity events can happen far before convincing clinical data, biotechs are incentivized to push their risky studies until as late as possible (ideally after the employees get their money) and to gussy them up to look better than they really are. A fail-fast-and-publish-honestly mentality would of course be better for longevity overall.
Then again, it’s not like academic research is necessarily better. Medical research results in general are apparently unreproducible up to 50% of the time. Plus private biotech can afford to do many things that academic labs can’t. Fortunately, some private funders are working to fill this gap.
At the regulatory level
Before it can conquer death, a longevity treatment will have to conquer the U.S. FDA’s clinical trial process, at least if it wants to become part of the mainstream U.S. healthcare system. The FDA doesn’t consider aging to be a medical indication (a.k.a. something a company can advertise a medicine to fix). This means longevity companies have to choose a specific age-related indication like Alzheimer’s against which to demonstrate their treatment’s efficacy.
More optimistically, regulatory changes like dual-track clinical trials or eliminating phase 3 trials altogether might entail a massive acceleration in longevity therapeutic development. Outsourcing or decentralizing clinical trials is also an exciting option. Science 37 is one company working on this.
It’s also possible companies could avoid the FDA altogether, though it’s not clear how this would go down in practice. A company can sell therapeutics-in-all-but-name as supplements or general wellness devices without getting FDA approval for them, and doctors can prescribe more-or-less anything off-label, but the FDA can, and often does, decide to crack down on such gray-area activities. Another option in the near term is focusing on longevity in pets as are Rejuvenate, Loyal, and GenFlow. This may be a great way to get a lot of good science done with relatively less regulation before trying to clear the FDA hurdles.
At the macro level
The high costs and failure rates of drug development make it a tough space for entrepreneurial disruption. (The first chapters here are illustrative.)
This is partly because high risk makes behavior change hard. There’s natural resistance to changing any part of the playbook. As one medicinal chemist put it to me “if you make a service 2x better, nobody cares. If you make it 10x better, or tell them to try a new approach, they won’t believe you. 4x or 5x better with their existing process is optimal.” Not exactly culture of disruptive innovation. One exception is the genetic sequencing sector, which has been extremely disruptive and innovative. I’d like to better understand what degree of price decrease and reliability was required for that.
Another reason biotech entrepreneurship is hard is that the payoffs take so long to arrive, way after all the research is done. This means platforms and services for drug development aren’t bought and sold by biotechs as much as bartered for with equity in whatever future drugs they hopefully produce. And this in turn makes it more complicated and expensive to start companies offering platforms and services to biotechs compared to other industries.
As if all the above wasn’t good enough, the time and cost required to make a drug are increasing over time, despite lots of technological advancement. There’s even a cute name for it: Eroom’s law. Nobody knows why it’s happening, but if the causes of Eroom’s law are exogenous, we should launch more longevity therapeutic ventures sooner while it’s relatively cheaper.
So, which has more marginal benefit, improving the drug discovery system or going after longevity with the existing system? Obviously I have no idea, but the point is that it’s not obviously the latter. We should also appreciate how many important problems there are to be solved outside the lab and with non-biology skills.
Externalities
The pursuit of longevity doesn’t happen in a vacuum. A full answer to “should we pursue human longevity” needs to take all externalities into account.
A big externality is the aspirational value of longevity as a brand/meme for biotech in general. Some might argue that society is already working on longevity with every new medicine it develops. If we keep curing things that kill us, eventually we stop dying, right? Who needs the new branding? Well, a lot of longevity investment is not coming from traditional biotech/pharma investors, but from Silicon Valley. That may be attributable to the aspiration value. And even if the aspirational call of longevity does nothing more than encourage talented people to work on biotech, that may be well worth it.
Another possibility is that the promise of death-postponement could spur funding increases or regulatory reforms for drug discovery. This is an advantage of the aforementioned efforts focused on longevity in charismatic species like household pets. If everyone’s dogs were suddenly living to age 30, people would hopefully start getting jealous.
On the other hand, if a field over-promises and under-delivers it can go through a winter. This wasn’t great for the field of AI, and it would be nice if longevity can avoid this. There’s also brand risk. Gene therapy was delayed a lot by the death of a teenager in 1999. Psychedelics were delayed by 50+ years after society turned against them, despite being widely accepted among the wealthy and well-educated in the mid-20th century. If longevity therapies develop brand recognition — I’m looking at you, senolytics — and there’s a major adverse incident, or even just embarrassments, it might have been better to keep a low profile.
Then again, the same fears of death that make longevity prone to pseudoscience might act as a countervailing force to a winter. People don’t really care about AI research. They do care about staying alive.
Opportunity Costs
Why do we care about longevity? Because death is bad.4 There are four problems with it:
- You suffer through aging and dying
- Others suffer as they empathize and care for you
- It precludes future utility for you
- It precludes future utility for others, including the loss of your contributions to society
But longevity isn’t the only solution to these problems. And these problems aren’t the only causes of suffering in the world that we might care about. This means we have two opportunity cost calculations to make.
Is longevity the best solution to these problems?
Humanity has made a massive dent in problems 1 and 2 with analgesics. Analgesics get short shrift in the history of technology, but they’re one of the greatest medical victories ever won. They also have lots of room for improvement, though that improvement may be hard to attain if the history of analgesic development is any indication. Then again, it’s hard to imagine it being any harder than longevity therapeutic development.
Physical pain is hardly the only kind of suffering implicated in problems 1 and 2, of course. Aging and death, and witnessing them, entail significant existential suffering and anguish. (Citation probably unnecessary.) If the psychonauts are to be believed, we may be close to treatments for those, too: clinical results so far are promising. But it’s hard to imagine psychedelics making us fully disinterested in death. Doing so would require significant improvements to analgesia, neuromodulation techniques, and cultural attitudes toward medication and death.
Some readers may feel that pain- or mood-manipulation are inauthentic ways to defy death. I’d gently remind such readers that we’re comparing these strategies against making humans immortal, so you may want to check your naturalistic fallacy at the door.
Problems 3 and 4 run up against hairy philosophical issues about the continuity of an individual life and whether it has inherent value. As a personal diagnostic, you can ask yourself whether you think one person living 100 healthy years is different to two people living 50 healthy years each, all things equal. If you think they’re the same, and you’re not an antinatalist, problems 3 and 4 aren’t problems for you at all.
Are these the best problems to work on?
I hate this question, because the right answer to “should we fund longevity or something else?” is “can we seriously not fund both?!” It’s also suspiciously close to the fallacy of relative privation, which longevity detractors fall prey to whenever they argue against longevity research in principle due to the existence of other big problems in the world.
But the fallacy of relative privation isn’t a fallacy when making a cost-benefit analysis — something defenders of longevity can forget. Society does have to make choices about where it spends its resources. Even if its choice is to spend them on tokenized digital cats.
I’ll leave debating those choices to the experts. And what you should work on comes down to personal fit. Emanuele Ascani, Matthew Barnett, and their commenters have gotten some nice conversations started on the topic. But suffice it to say there are no decisive arguments that longevity research is more or less important than, say, existential risk research, or promoting pronatalism.5 Unless, that is, you have some unusual conceptions of value being inherently tied to human lifespans and not human lived experience itself.6
Conclusions
We shouldn’t overestimate how much we understand aging, and we shouldn’t underestimate how much progress we can make without full understanding.
On the margin I agree with Open Phil that basic research and engineering for biotech tools are likely to have the biggest impact on longevity, whether this research is called “longevity research” or not. I’d say this is especially true for tools like biomarkers to enable large-scale phenotypic assays. The hard(er) part of building the atomic bomb wasn’t the nuclear physics, it was building the bomb, and I suspect longevity is similar.
There are a million problems to be solved in the larger biotech ecosystem that will help achieve human longevity. Solving them may accomplish more for longevity than any direct work on aging at this point.
Many problems in the larger biotech ecosystem don’t require primary expertise in biological science. And most of them aren’t specific to longevity and require no explicit association with longevity. This is useful for decoupling longevity progress from the longevity brand.
Despite how underrated longevity research is, neuro- and bio-technologies addressing suffering at its source in consciousness and the brain are underrated even more, despite the tech world’s recent interest in psychedelic therapy and pharma’s historic interest in non-addictive painkillers. Neuromodulation, mood adjustment, and new forms of analgesia should be getting as much or more attention than longevity.
Have feedback? Find a mistake? Please let me know!
Many thanks to Egan Peltan, Stephen Malina, Gavin Taylor, Alexey Guzey, Alex K Chen, William Eden, Karl Pfleger, Nathan Cheng, and Prof. João Pedro Magalhães for conversations and feedback.
And an extra huge thank you to Sarah Constantin and Elizabeth Van Nostrand (whom you can and should hire to do research, by the way) for their help.
Appendix: Bad arguments against human longevity
- “Death makes life meaningful”
- You could make the same argument about the opposite of any good thing (we need pain to appreciate pleasure, etc.). This is not usually considered grounds for not working on them.
- People will still die from accidents, so the “thrill of living” will still exist.
- It might even increase, since unexpected death would interrupt the grander plans of those who expecting to live forever.
- You can always opt into death.
- “People will just suffer forever”
- A.k.a. the Tithonus Error. Nobody’s talking about this when they talk about longevity.
- It is true though that there’s no guarantee a longevity therapy will increase healthspan rather than just increasing morbidity, so care should be taken here.
- “People will get bored,” or “society will stagnate”
- Again, anyone’s welcome to get off the ride anytime they want.
- Inequality: “Some people will get treatments first”
- We already live like this, just compare lifespans across countries.
- A net increase in wellbeing is worth doing, even if it’s unequally distributed.
- It’s important to factor in the negative wellbeing associated with being on the losing end of an unequal split, but still net positive.
- “Incumbents won’t ever die”
- Already a problem in the real world w.r.t. regimes instead of people. Longevity doesn’t materially change this.
- “Something something Malthus”
- The worst versions of these arguments go “if nobody dies, our population will explode, and we’ll all starve to death!”
- This could only happen if we developed true immortality. Otherwise is just slightly increases the population, since people are dying at the same rate, just later.
- If we do develop true immortality, we would also almost certainly have the technology to be fertile at any age. (Not to mention sufficient technology to feed everyone.) This would reduce the urgency to have children in the unlikely case of a Malthusian scenario.
- There are more realistic ones though (perhaps not technically Malthusian). Suppose a senolytic cocktail was approved in 2040 that extended human lifespan by median 50 years but didn’t ameliorate various nonterminal dementias, which set in at 100. And suppose these dementias resisted treatment until 2050, 2060, or later. This future would likely be net positive for the world, but practically speaking it would indeed amount to an economic and ethical crisis.
- The worst versions of these arguments go “if nobody dies, our population will explode, and we’ll all starve to death!”
- “You can’t go to heaven if you don’t die”
- The sorts of secular people who write articles promoting longevity research always seem to leave this one out.
- Nevertheless, if there’s a heaven (or hell) awaiting you, you’ll get there eventually. What’s the rush?
- “People just should die”
- This is the naturalistic fallacy. Apply Bostrom’s reversal test.
- Other, better rebuttals:
Notes
-
Herein I’m using the word “longevity” to mean increasing maximum (not median) human lifespan by an undeniable amount, whether that’s a 5 year increase or living indefinitely. Not that I’m not interested in increasing healthspan or median lifespan — especially considering those are much more measurable endpoints on the way to achieving greater longevity. Just being clear on terms. ↩︎
-
Yes, the Ship of Theseus does rear its bow here: it’s not clear the extent of interventions you can make to the human body while still leaving it “human.” With enough technology you can make a fish walk on land. But if you have to replace 50% of its genome with moose DNA and give it cyborg hooves to do it, is it still a fish? Philosophy is fun. ↩︎
-
On the bright side, there are about 50 companies pursuing NMEs (new molecular entities) for aging-related indications right now. So on priors we should expect a drug for an aging-related indication to be approved sometime within the next 10 years! ↩︎
-
Pronatalism seems like something longevity advocates should either be advocating for really hard or railing against. Either you think death is so monstrous that it makes life not worth living, on balance, and thus people need to stop having kids until we solve it (unless the idea is to draft these kids into the fight-longevity campaign); or you think life is great, which presumably is why you want to extend it, and thus we should make sure there’s more of it in the world, including by making more people. The only thing that can’t be right — unless you have a very bespoke person-affecting view — is having just 2.1 kids, whose lives are effectively a continuation of yours and your partner’s except punctuated by the tragedy of your deaths. ↩︎
-
My complaint is that this argument relies on a circular definition of scarcity as scarcity of time (“lifespans”) and not scarcity of things people value (“life”). Under the argument in the piece, living forever in immense suffering would count as success. ↩︎